| Accession: | |
|---|---|
| Functional site class: | PDZ domain ligands |
| Functional site description: | The best characterised PDZ ligands (PBMs, PDZ-Binding Motifs) are short C-terminal peptides that bind in a surface groove of PDZ domains of proteins as a part of a variety of biological processes including cell signalling and synapse. Although there is a considerable literature on internal sequence peptide interactions, we are not currently representing internal PDZ-binding peptides in ELM. |
| ELMs with same func. site: | LIG_PDZ_Class_1 LIG_PDZ_Class_2 LIG_PDZ_Class_3 LIG_PDZ_Wminus1_1 |
| ELM Description: | PDZ domains recognize short sequences at the carboxy terminus of target proteins. The terminal residue is apparently always hydrophobic with the -2 position being a strong determinant of specificity. The class 1 motif has a pattern such as (ST)X(VIL)*. We have made the terminal position more relaxed based on experimental binding data. However, probably not all PDZ domain instances can accept either A or F. Several less conserved positions in the motif may modulate affinity and specificity of the ligand domain interaction. |
| Pattern: | ...[ST].[ACVILF]$ |
| Pattern Probability: | 0.0000725 |
| Present in taxons: | Eukaryota Homo sapiens Metazoa Mus musculus |
| Interaction Domain: |
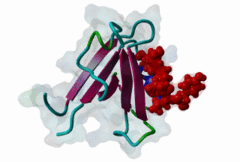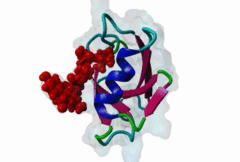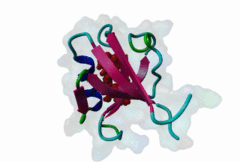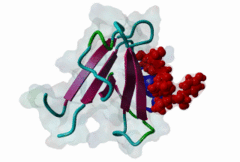
PDZ (PF00595)
PDZ domain (Also known as DHR or GLGF)
(Stochiometry: 1 : 1)
|
PDZ domains are ~90 residue globular protein modules that can be found in eukaryotic regulatory proteins. They are found in most eukaryotes but the domain family is hugely expanded in the Metazoa (over 250 PDZ domain instances are found in the Human proteome). Thus nearly all of the metazoan PDZ domain functions will be specific to this evolutionary lineage. Most PDZ domain-containing proteins spend at least part of their time in membrane-associated complexes. Many PDZ ligands are themselves membrane proteins. Several PDZ domain containing proteins include multiple domain copies (MPDZ/MUPP1 and MAGI2 contain 13 and 6 PDZ domain instances respectively), acting as scaffolds, recruiting multiple PDZ domain binding proteins and facilitating the construction of large membrane-associated complexes. PDZ domains also co-occur regularly with other signalling/regulatory domains regulating many biological processes such as transport and signal transduction (Lee,2010). There is increasing evidence that some, ultimately perhaps most, PDZ domains also bind to phospholipid headgroups (Gallardo,2010). Thus PDZ domains have a role in assembly of signalling complexes at membrane locations determined by the appropriate lipid modification state. In large multiprotein complexes, they may do this in conjunction with other lipid-sampling domains such as C2, PH, PX and so forth to differentiate different membrane contexts. Peptide and lipid binding by PDZ domains has been found to be co-operative, enabling tight integration of lipid and protein regulatory signals. For the carboxy-terminal binding motifs, position 0 (assigned to the C-terminal residue) is always hydrophobic while the class is determined by the position -2 (third last) residue. These two positions are the most buried in the bound complexes, hence they are strong specificity determinants. The sidechains of the ligand residues interact with a neighbouring α-helix in the PDZ domain, while the backbone of the ligand binds to the PDZ domain via β augmentation: it forms a β-strand with a neighbouring β-sheet in the domain (Chi,2012, Luck,2012). This mode of interaction is the most known but it doesn’t cover all PDZ binding motifs. For instance, the specificity of a PDZ ligand can also be defined by a tryptophan in position -1, which forms a hydrophobic interaction with the residues in the neighbouring β-sheet in the domain, without forming a β-strand. Apart from ligand positions 0, -1 and -2 being strong determinants of specificity, the neighbouring residues can undoubtedly contribute to specificity and affinity of the interactions (Ernst,2014). In ELM we have chosen relaxed motif patterns based on the solved PDZ complexes, so it is likely that any given peptide will only bind to a subset of the PDZ domains belonging to that class. |
(click table headers for sorting; Notes column: =Number of Switches, =Number of Interactions)
Please cite:
ELM-the Eukaryotic Linear Motif resource-2024 update.
(PMID:37962385)
ELM data can be downloaded & distributed for non-commercial use according to the ELM Software License Agreement
ELM data can be downloaded & distributed for non-commercial use according to the ELM Software License Agreement