| Accession: | |
|---|---|
| Functional site class: | Cyclin N-terminal Domain Docking Motifs |
| Functional site description: | Cyclin-dependent kinases (Cdks) coordinate hundreds of molecular events during the cell cycle via Ser/Thr phosphorylation. With cell cycle progression, different cyclins bind to Cdks to control their function by providing docking sites for substrates and also by modulating Cdk active site specificity. Docking motifs control the timing of cell cycle events by enabling preferential interaction and phosphorylation of substrates by a specific cyclin/Cdk complex. Cyclins use the conserved hydrophobic pocket (hp) to bind docking motifs on partner proteins. In the budding yeast, the divergence of the hp has given rise to a family of related RxL-like docking motifs consisting of a hydrophobic core modulated by positively charged (RxLF, RxLxF) or hydrophobic (LxF, PxF, NLxxxL) residues. Cyclins may use additional surfaces to dock substrates, as with the mammalian Cyclin D-specific (DOC_CYCLIN_D_Helix_1) and the budding yeast Cln2-specific leucine- and proline-rich LP (DOC_CYCLIN_yCln2_LP_2) motifs. |
| ELMs with same func. site: | DOC_CYCLIN_RevRxL_6 DOC_CYCLIN_RxL_1 DOC_CYCLIN_yClb1_LxF_4 DOC_CYCLIN_yClb3_PxF_3 DOC_CYCLIN_yClb5_NLxxxL_5 DOC_CYCLIN_yCln2_LP_2 |
| ELM Description: | The classical RxL cyclin recognition motif is found in a wide range of cyclin/CDK interacting proteins (Wohlschlegel,2001; Schulman,1998) including phosphorylation targets like p53, pRb, E2F, p107 (1H24; 1H25; 1H26; 1H28), and CIP-KIP family Cdk inhibitors (1JSU; 6P8E; 6P8H; Russo,1996; Wohlschlegel,2001; Guiley,2019). The presence of this docking motif substantially increases the level of phosphorylation of Cdk substrates at ([ST])Px(0,2)[KR] motifs (MOD_CDK_SPxK_1; Takeda,2001). It is highly conserved in eukaryotes. Several yeast Clb5 substrates employ RxLs for docking as do those of mammalian Cyclin A (Loog,2005; Koivomagi,2011). Cyclins show cross-specificity, for instance Cyclins E and D also bind RxL motifs (Guiley,2019). The classical cyclin docking motif pattern is mainly derived from peptides bound to Cyclin A as there are several complex structures available. Although the motif is often called RxL, there are actually four core binding residues, with only the Leucine being fully conserved. The motif binds in a hydrophobic groove with charged residues lining the edge. Peptide backbone hydrogen bonds guide the four core binding residues into the groove. There is a clear but non-essential preference for basic residues preceding the core motif and for acidic residues following the core motif. The first core binding position is quite shallow, accepting either hydrophobic or basic residues. It is followed by a residue facing outwards, which cannot accept the short acidic residue Asp. The next residue lies in a pocket and must be either Arg or Lys. It is followed by a residue facing outwards, which cannot accept the short acidic residue Asp. Then comes the Leu residue fitting into the hydrophobic groove. Flexible spacing then allows one optional externally facing residue. The final core hydrophobic residue is one of Phe, Pro, Leu or Met. The derived regular expression pattern captures the core motif and approximates the weaker charge preference to either side. |
| Pattern: | (.|([KRH].{0,3}))[^EDWNSG][^D][RK][^D]L.{0,1}[FL].{0,3}[EDST] |
| Pattern Probability: | 0.0018793 |
| Present in taxons: | Eukaryota Viruses |
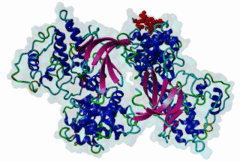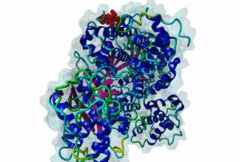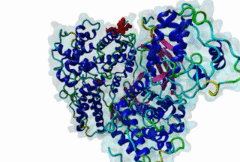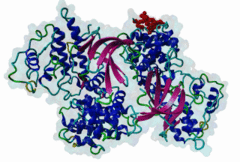
| Interaction Domain: |
Cyclin_N (PF00134)
Cyclin, N-terminal domain
(Stochiometry: 1 : 1)
|
Cyclin-dependent kinases (Cdks) are central regulatory enzymes of the eukaryotic cell cycle. The sequential attachment of different cyclins to Cdks represents the periodic driving force that ensures a controlled progression through the cell cycle. Although there can be functional overlap, the various cyclin/Cdk complexes are specialized for optimum performance of discrete tasks. The cell cycle of the budding yeast Saccharomyces cerevisiae is remarkably simplified compared to that of mammalian cells and therefore it was the subject of many cell-cycle related studies and is currently better understood. Here a single Cdk, Cdk1, associates with different cyclins to mediate all major cell cycle transitions. Cyclins Cln1–3 are triggers for G1 and G1/S, while among B-type cyclins Clb5 and Clb6 drive S phase, Clb3 and Clb4 are specific for early mitotic events, and Clb1 and Clb2 complete the progression to mitosis. Detailed analyses of the budding yeast cell cycle provide important clues on the mechanisms that allow the fine-tuning of thresholds and the ordering of the switch points that drive cell cycle events. These mechanisms rely strongly on the linear encoding of SLiMs to direct cell cycle phosphorylation events (Ord,2019). Limited evidence suggests that these mechanisms have parallels in mammalian cyclin-Cdk regulation. Cyclins from yeasts and animals harbour a highly conserved surface patch called the hydrophobic pocket (hp) that recognizes docking motifs on partner proteins (DOC_CYCLIN_RxL_1; Loog,2005). The RxL docking motif mediates binding to the hp of a broad range of cyclins from budding yeast (Clb1-6) and mammalian cells (cyclins D/E/A/B). Studies in budding yeast have identified more specific motifs that target the hp. For example, G2 cyclin Clb3 recognizes substrates with the PxF motif (DOC_CYCLIN_yClb3_PxF_3; Ord,2020), and when Cdk is coupled to mitotic cyclins Clb1 or Clb2, the resulting M-Cdk complex recognizes the LxF motif (DOC_CYCLIN_yClb1_LxF_4; Ord,2019). Likewise, the NLxxxL motif is homologous to RxL, but has evolved exclusive specificity for S-phase cyclins Clb 5/6 (DOC_CYCLIN_yClb5_NLxxxL_5; Faustova,2021). Other cyclin-specific motifs include the leucine- and proline-rich LP docking motif (DOC_CYCLIN_yCln2_LP_2; Koivomagi,2011; Bhaduri,2011), which directs binding to late G1-cyclins Cln1/2. Specific docking motifs are also present in mammalian cyclins, as with the cyclin D-specific helical docking motif (DOC_CYCLIN_D_helix_1; Topacio,2019) that mediates binding of Rb proteins to Cyclin D to drive the G1/S transition. Cyclin docking motifs are not only employed by substrates, they are also frequently employed by regulators of cyclin/Cdk complexes, for example the mammalian p27Kip1 and p21Cip1 cyclin inhibitors (1JSU; 6P8E, 6P8H) which hide the site from substrates or the yeast Swe1 that keeps M-CDK in an inactive state during earlier phases of the cell cycle (Ord,2019). The differences in specificity of hp-docking motifs are explained by changes in the residues that make up the 210-MRAILVDW-217 helix in the hydrophobic pocket (numbering according to human cyclin A2) (Ord,2019). The structures of several RxL motifs (p53, pRb, E2F, and p107) bound to cyclin A2 (1H24; 1H25; 1H26; 1H28) reveal that the central R/K residue of the RxL motif hydrogen bonds to E220 in cyclin A2, while two hydrophobic/aromatic positions bind to an apolar groove made up by M210, I213, L214 and W217 (Russo,1996; Lowe,2002). Residues surrounding the hp (E224 and R250) shape its charge specificity and determine a preference for basic or hydrophobic residues in the vicinity of the core motif. In the budding yeast, loss of the acidic E220 residue in G2- and M-phase cyclins weakens their preference for RxL sequences, favouring the emergence of related (PxF and LxF) motifs that preserve the hydrophobic mode of interaction (Bhaduri,2011; Ord,2019; Ord,2020). Similar changes in the hydrophobic pocket of mammalian cyclins make M-cyclin (Cyclin B) a poor binder of RxL motifs. Early cyclin/Cdk complexes have low intrinsic activity toward the optimal substrate motif compared to the potent mitotic Cdks, still they need to initiate such important events as Start and S phase. The cyclin-specific docking sites described above are able to compensate for the gradually decreasing specificity of early cyclin/Cdk complexes (Loog,2005; Koivomagi,2011; Bhaduri,2011; Bhaduri,2015; Ord,2019; Ord,2019). Also, cyclins are not just activators of Cdk1 but are also modulators of the catalytic specificity of the kinase active site (Koivomagi,2011). Therefore, modulation of Cdk1 active-site substrate specificity combined with cyclin-specific docking enables regulated changes in Cdk1 specificity and provides a wide range of selective switch points that drive cell cycle transitions (Koivomagi,2011). Mammalian cyclins might use similar mechanisms to ensure specific substrate docking at different stages of the cell cycle, but hp-docking motifs different from the canonical RxL sequence remain to be elucidated with one exception, the reverse RxL motif in Skp2 (DOC_CYCLIN_RevRxL_6; Kelso,2021). |
(click table headers for sorting; Notes column: =Number of Switches, =Number of Interactions)
Please cite:
ELM-the Eukaryotic Linear Motif resource-2024 update.
(PMID:37962385)
ELM data can be downloaded & distributed for non-commercial use according to the ELM Software License Agreement
ELM data can be downloaded & distributed for non-commercial use according to the ELM Software License Agreement