LIG_FHA_2
| Accession: | |
|---|---|
| Functional site class: | FHA phosphopeptide ligands |
| Functional site description: | The FHA domain is a signal transduction module which recognizes phosphothreonine containing peptides on the ligand proteins. FHA domains partake in many signalling processes but are especially prevalent in nuclear proteins that are involved in cell cycle checkpoint, DNA repair and transcriptional regulation. |
| ELMs with same func. site: | LIG_FHA_1 LIG_FHA_2 |
| ELM Description: | LIG_FHA_2 motifs are short phosphothreonine peptide modules contains acidic amino acids at the pT+3 position. The motif has the consensus sequence of T..[ED]. FHA domains with this preference are found in checkpoint/repair proteins MRC1 and Rad9 of Fungi and Metazoa Xrcc1 (Luo,2004) and Xrcc4 (Koch,2004). |
| Pattern: | ..(T)..[DE]. |
| Pattern Probability: | 0.0082864 |
| Present in taxon: | Eukaryota |
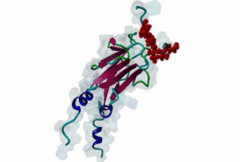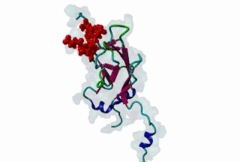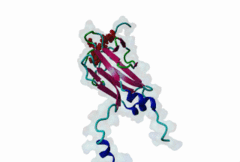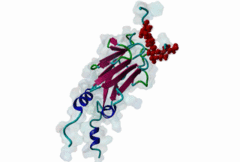
| Interaction Domain: |
FHA (PF00498)
FHA domain
(Stochiometry: 1 : 1)
|
The forkhead-associated FHA domain is a phosphopeptide-binding domain first identified in a group of forkhead transcription factors (Hofmann,1995). FHA are small domains (<100 amino acids) that form a sandwich of two anti-parallel beta sheets. They are present in a wide variety of proteins from both prokaryotes and eukaryotes (Li,2000). The existence of FHA domains in a wide variety of proteins means they are involved in diverse cellular functions including signal transduction and vesicular transport. In plants, FHA domains participate in the regulation of receptor-like protein kinase signalling pathways (Lee,2003). There are many nuclear FHA-domain containing proteins: these have a variety of roles involved in cell-cycle checkpoint control, DNA repair, signal transduction, transcriptional regulation, and pre-mRNA splicing. Some of the FHA domain-containing proteins are present in the plasma membrane. While FHAs bind to phosphothreonine motifs, BRCT domains recognize phosphoserine motifs in otherwise similar nuclear regulatory contexts. Although weak in vitro binding of phosphoserine and phosphotyrosine peptides has been observed, all high affinity interactions utilize phosphothreonine, which may be an essential requirement for the biological ligands. The optimal FHA domain binding sequence is a phosphothreonine peptide with pT+3 specificity. So far there are two well characterised motifs: TXX[ILV] and TXX[DE]. While TXXC and TXXA have been observed, they are not currently modeled in ELM. The TXXC linear motif forms part of a larger induced fit interaction with the FHA domain (Li,2000; Yongkiettrakul,2004; Lee,2003). More variations among the FHA-binding motifs are expected to be found. |
-
Solution structures of two FHA1-phosphothreonine peptide complexes provide
insight into the structural basis of the ligand specificity of FHA1 from
yeast Rad53.
Yuan C, Yongkiettrakul S, Byeon IJ, Zhou S, Tsai MD
J Mol Biol 2001 Nov 30; 314 (3), 563-75
PMID: 11846567
-
Replication checkpoint protein Mrc1 is regulated by Rad3 and Tel1 in
fission yeast.
Zhao H, Tanaka K, Nogochi E, Nogochi C, Russell P
Mol Cell Biol 2003 Nov; 23 (22), 8395-403
PMID: 14585996
-
Xrcc4 physically links DNA end processing by polynucleotide kinase to DNA
ligation by DNA ligase IV.
Koch CA, Agyei R, Galicia S, Metalnikov P, O'Donnell P, Starostine A, Weinfeld M, Durocher D
EMBO J 2004 Oct 1; 23 (19), 3874-85
PMID: 15385968
-
The molecular architecture of the mammalian DNA repair enzyme,
polynucleotide kinase.
Bernstein NK, Williams RS, Rakovszky ML, Cui D, Green R, Karimi-Busheri F, Mani RS, Galicia S, Koch CA, Cass CE, Durocher D, Weinfeld M, Glover JN
Mol Cell 2005 Mar 4; 17 (5), 657-70
PMID: 15749016
(click table headers for sorting; Notes column: =Number of Switches, =Number of Interactions)
| Acc., Gene-, Name | Start | End | Subsequence | Logic | #Ev. | Organism | Notes |
|---|---|---|---|---|---|---|---|
| P14737 RAD9 RAD9_YEAST |
153 | 159 | LRKKMTFQTPTDPLEQKTFK | TP | 2 | Saccharomyces cerevisiae (Baker"s yeast) | |
| P14737 RAD9 RAD9_YEAST |
190 | 196 | EENASLEVTEADATFVQMAE | TP | 2 | Saccharomyces cerevisiae (Baker"s yeast) | |
| P18887 XRCC1 XRCC1_HUMAN |
521 | 527 | YAGSTDENTDSEEHQEPPDL | TP | 2 | Homo sapiens (Human) | |
| Q9P7T4 mrc1 MRC1_SCHPO |
643 | 649 | SQVDSLVPTQLDSTIPTQID | TP | 2 | Schizosaccharomyces pombe (Fission yeast) | |
| Q13426 XRCC4 XRCC4_HUMAN |
231 | 237 | RDPVYDESTDEESENQTDLS | TP | 4 | Homo sapiens (Human) | |
| Q924T3 Xrcc4 XRCC4_MOUSE |
229 | 235 | EHGLYDGSTDEESGAPVQAA | TP | 1 | Mus musculus (House mouse) |
Please cite:
ELM-the Eukaryotic Linear Motif resource-2024 update.
(PMID:37962385)
ELM data can be downloaded & distributed for non-commercial use according to the ELM Software License Agreement
ELM data can be downloaded & distributed for non-commercial use according to the ELM Software License Agreement