| Accession: | |
|---|---|
| Functional site class: | 14-3-3 binding phosphopeptide motif |
| Functional site description: | The 14-3-3 proteins are a family of conserved regulatory molecules that are involved in diverse cellular processes through the interaction with hundreds of different proteins. In mammals, seven isoforms are present. 14-3-3 proteins form either homo- or heterodimers that target certain phosphoserine/threonine-containing motifs with a low micromolar affinity. Binding to a small set of unmodified proteins has also been reported. Phosphorylation-dependent and independent binding occurs via the same deep ligand-binding groove. There are canonical arginine-containing motifs and a non-canonical motif group that are difficult to classify but utilize additional hydrophobic interactions. The canonical Arg-containing 14-3-3 binding peptides are phosphorylated by members of basophilic kinases. Sites phosphorylated by Proline-directed kinases cannot be bound by 14-3-3 proteins, hence there is no overlap with the basophilic kinase signalling pathways. |
| ELMs with same func. site: | LIG_14-3-3_CanoR_1 LIG_14-3-3_ChREBP_3 LIG_14-3-3_CterR_2 |
| ELM Description: | The canonical motif has at least one Arg residue located at the -4,-3 or -2 position relative to the phospho-residue which aids in the placement of the peptide in the binding groove. Arg stabilizes the phospho-peptide conformation by forming hydrogen bonds as well as an intramolecular salt bridge with the phosphate group. Asp/Glu are not tolerated for at least 3 positions preceding the phosphorylated residue. Pro and Gly at position -1 are also not tolerated since they weaken the interaction between the phospho-residue and the 14-3-3 binding pocket. Proline is also disallowed at position +1 (Panni,2011). At this position, the -NH group of the peptide backbone forms a hydrogen bond with the 14-3-3 protein as part of local geometry crucial to orientate the adjacent phosphorylated sidechain (Molzan,2012). Therefore, there is no physical space for a Pro. The canonical binding motif requires at least one favourable hydrophobic interaction following the P-site residue as this part of the 14-3-3 groove is markedly hydrophobic. This residue can be located at three different distances following the phosphosite. These alternatives arrangements are non-exclusive. In the first option, the +1 residue is a bulky hydrophobic residue with an extensive packing face (5D3E, 4DAU). If there is no hydrophobic residue at +1, other residues are tolerated with the exception of the disallowed Arg, Ile, Gly, Lys and Asn (Panni,2011). In the second option, there is a Pro at +2. Pro is strongly favoured in the +2 position as it introduces an exit kink into the peptide chain and contacts hydrophobic walls of the groove (3O8I, 3MHR, 2C74, 2BR9, 2BTP, 3UAL, 4IEA, 4IHL). If option 1 or 3 are not present, option 3 is a requirement for a bulky hydrophobic residue in the +4 to +6 range which loops back down into the hydrophobic wall of the groove (5D2D, 4WRQ, 2C1J). Variant 2 and 3 have been reported more often than variant 1. |
| Pattern: | R[^DE]{0,2}[^DEPG]([ST])(([FWYLMV].)|([^PRIKGN]P)|([^PRIKGN].{2,4}[VILMFWYP])) |
| Pattern Probability: | 0.0044767 |
| Present in taxon: | Eukaryota |
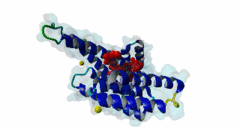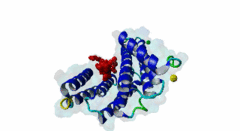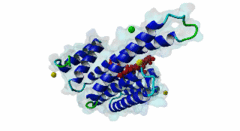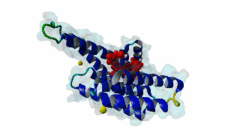
| Interaction Domain: |
14-3-3 (PF00244)
14-3-3 protein
(Stochiometry: 1 : 1)
|
The 14-3-3 proteins (PF00244) are a highly conserved group of proteins, present in most or all eukaryotic organisms. In the mammalian proteome seven isoforms can be found, whereas only two isoforms are present in yeasts, insects and nematodes (Obsil,2011). 14-3-3 proteins can form both homo-and heterodimers (1A4O, 5IQP). Each monomer consists of nine antiparallel alpha-helices with a deep central binding cavity that can accommodate peptides from a multitude of diverse binding partners. Consequently, 14-3-3 proteins are shown to be involved in important cellular processes such as signal transduction, cell-cycle control (Peng,1997), apoptosis (Zha,1996), stress response and malignant transformation (Darling,2005). The 14-3-3 proteins posses no catalytic activity and thus they perform their functional tasks only by binding and modulating the activity of their partner proteins. Mechanisms of 14-3-3 action include the induction of conformational changes, the occlusion of sequence-specific or structural features, scaffolding complexes (Bridges,2005) and change of cellular localization (Obsilova,2005). All 14-3-3 isoforms primarily target phosphoserine/-threonine peptide motifs in their interaction partners. The phosphate group orients the peptide and is stabilized by a conserved 14-3-3 Arg-Arg-Tyr triad forming a positively charged pocket within the otherwise amphiphatic 14-3-3 ligand groove (Yang,2006): It is unlikely that phosphomimetic residue substitutions will function due to the number and orientation of the charged functional groups binding the phosphate. Phosphopeptide library screens and crystal structures led to the definition of consensus motifs such as RSxpSxP, RxxxpSxP and pS/pTx1-2-COOH used to classify the number of 14-3-3 mediated interactions (Muslin,1996, Yaffe,1997). The experimental data of Panni et al., 2011 (Panni,2011) are helpful for revising the motif description, including where certain residues are disallowed. In the current ELM entry, we have been able to define a canonical Arg-containing motif where the phosphoresidue is followed by one or more bulky hydrophobic residues with several preferred spacings, and a second shorter C-terminal Arg-containing motif, terminating just after the phosphoresidue. Both motifs are observed in numerous experimentally validated examples, and there are many crystal structures of phosphopeptide/14-3-3 complexes. Since at least one positively charged arginine precedes the phosphorylated residue, these canonical motifs are compatible with phosphorylation by the very large group of basophilic Ser/Thr kinases including PKA, CaMKII and the Aurora kinases. The blocking effect of Pro at +1 excludes14-3-3 proteins from binding substrates of proline-directed kinases (e.g. MAPK, CDK), even when these are preceded by an Arg residue. Many 14-3-3 binding proteins such as Raf-1 contain several 14-3-3 binding sequences, separated by variable length polypeptide segments (4IHL). Binding studies have shown that a 14-3-3 dimer can cooperatively bind two phosphorylated epitope sites on the same target protein partner. For ADAM22, Raf-1 and CFTR, dual binding resulted in higher affinity, more stable, complexes at which the space between the strong tandem 14-3-3 motifs averaged 14, 18 (4IHL) and 15 amino acids (5D2D, 5D3E), respectively (Yaffe,1997, Stevers,2016). As well as the classical canonical motifs, it has become clear that there are several varieties of non-canonical motifs, including unphosphorylated peptides. In some 14-3-3 binding partners, a second weaker motif (e.g. substituting Lys for Arg) is present in addition to a strong canonical motif. Examples are the proteins KSR1 (Cacace,1999), HDAC4 (Rose,2012, 3UZD), PADI6 (Rose,2012, 4DAT), MDM4 (Pereg,2006) and p53 (Schumacher,2010, 3LW1). A second non-canonical class of 14-3-3-binding motif lacks the basic residue but compensates with additional hydrophobic interactions using several residues following the phosphorylated residue. A number of such variants have been tested in vitro and bind with affinities equivalent to the canonical motifs, leaving little room for doubt that these interactions are biologically significant. Among 14-3-3 binding partners in which only a non-canonical hydrophobic motif was proved to mediate a good-affinity interaction are the integrins β2 (2V7D) and α4 (Bonet,2013) and plant H+ ATPases (Ottmann,2007, 2O98). As yet, we have not been able to define ELM motifs for the non-canonical hydrophobic 14-3-3 binding variants but it is important to be aware of their existence. Certain fungal toxins and bacterial virulence factors target 14-3-3 proteins. The fungal phytotoxin fusicoccin is shown to stabilize the protein complex between the plant plasma membrane H+-ATPase (PMA2) and 14-3-3 proteins (Wurtele,2003). Subsequent studies with semi-synthetic fusicoccanes describe the ability of fusicoccanes to enhance human 14-3-3 protein-protein interactions (Anders,2013, 3SMM, Stevers,2016, 5D3F). Although 14-3-3 interactors are overwhelmingly found to contain a phosphorylated residue, even this rule is not absolute. 14-3-3 proteins can also recognize a few unmodified peptides notably the pathogenic Pseudomonas exoS protein (Fu,1993, Yang,2006, 2C23). The exoS peptide LLDALDL binds in the reverse orientation compared to the phosphorylated peptides and therefore it is possible that there exist cellular equivalents of this binding mode (Ottmann,2007, 2O02). In addition, another helical peptide interaction is stabilized by Adenosine Monophosphate (AMP) in the case of the glucose responsive ChREBP transcription factor (Sato,2016, 5F74). The phosphate group of AMP occupies the 14-3-3 phosphate-binding site which is formed by an Arg-Arg-Tyr triad. |
(click table headers for sorting; Notes column: =Number of Switches, =Number of Interactions)
Please cite:
ELM-the Eukaryotic Linear Motif resource-2024 update.
(PMID:37962385)
ELM data can be downloaded & distributed for non-commercial use according to the ELM Software License Agreement
ELM data can be downloaded & distributed for non-commercial use according to the ELM Software License Agreement