| Accession: | |
|---|---|
| Functional site class: | FBP Nbox motif |
| Functional site description: | FUSE, FBP (Q96AE4) and FIR (Q9UHX1) comprise the core component of a system regulating the transition from transcription initiation through promoter escape and thereby an increase in the level of c-Myc (P01106) during cell cycle. Transcription of this proto-oncogene is controlled by the counter-balancing forces of two proteins on the transcription factor TFIIH: the FUSE binding protein (FBP) and the FBP-interacting repressor (FIR). The key interactions are mediated through the protein and DNA binding surface on the RRM1 (PF00076)-RRM2 (PF04059) tandem domains of FIR and the Nbox and KH domains (PF00013) present in FBPs. The Nbox is within a 26aa length segment in FBP family proteins which binds to the RRM2 domain of FIR. |
| ELM Description: | The N-Box, which is responsible for recruiting FIR (Q9UHX1) protein to the FUSE DNA sequence, is identified among FBP (Q96AE4) and some of its homologs. The N-box forms a helical conformation and docks on a hydrophobic surface defined by the RRM2 domain (PF04059) of FIR (Q9UHX1). The central patch of Alanine residues surrounded by larger hydrophobic residues are involved in the main interaction with RRM2. |
| Pattern: | F..A[ILV]..A..[ILV] |
| Pattern Probability: | 0.0000021 |
| Present in taxon: | Eukaryota |
| Interaction Domain: |
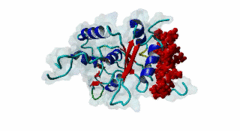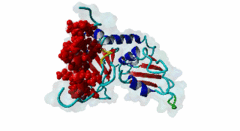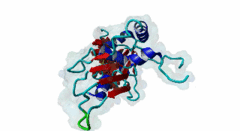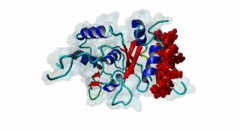
RRM_1 (PF00076)
RNA recognition motif. (a.k.a. RRM, RBD, or RNP domain)
(Stochiometry: 1 : 1)
|
Far upstream element (FUSE) Binding Proteins (FBPs) comprise an ancient family of single stranded DNA binding proteins which have different functions in gene regulation. They are originally identified as factors responsible for binding the AT-rich single-stranded DNA (ssDNA) sequence upstream of the P1 promoter of c-Myc (P01106) known as FUSE DNA (Duncan,1994). During the initiation of transcription FUSE starts to melt because of the torsional stress and is recognized by the FUSE binding protein (FBP) (Q96AE4). FBP is a stimulator of transcription factor TFIIH and thereby increases the transcription of c-Myc. The KH domains (PF00013) of FBP recognize the melted FUSE. On later stage an FBP-interacting repressor (FIR) (Q9UHX1) is recruited to the FBP-FUSE complex. It binds to both the FBP and FUSE, which is fully melted due to high level of DNA super-coiling. Upon binding to FBP-FUSE complex, FIR interacts with TFIIH reducing FBP mediated transcription and dissolution of the complex which brings the gene transcription to basal levels (Zhang,2013). A similar system of regulation of gene transcription is also observed in drosophila protein (d-myc) (Mitchell,2010). Among the FBPs, the FBP1 (Q96AE4) and FBP2 (Q92945) contain a specific region known as N-box, which mediates the recruitment of FIR through its RRM1 (PF00076)-RRM2 (PF04059) tandem domains (Cukier,2010). FIR recognizes the hydrophobic surface of the α helix formed by the N-box, while the hydrophilic surface does not seem to mediate the interaction. The KH domains (PF00013) present in the FBP proteins are also essential to recruit and retain FIR (Chung,2006). Apart from the regulation of gene transcription, FBPs take part in RNA binding, RNA trafficking, RNA editing, mRNA stabilization and degradation. The misregulation of the FBP-FIR-TFIIH is associated with many diseases. A splice isoform of FIR in human colorectal cancer tissue not only lacked the c-Myc suppressor action but also prevented the normal FIR from its function (Matsushita,2006). A defect in the operation of FBP-FIR-TFIIH is seen in patients with xeroderma pigmentosum B (XPB) (Liu,2006). |
(click table headers for sorting; Notes column: =Number of Switches, =Number of Interactions)
| Acc., Gene-, Name | Start | End | Subsequence | Logic | #Ev. | Organism | Notes |
|---|---|---|---|---|---|---|---|
| Q92945 KHSRP FUBP2_HUMAN |
74 | 84 | RKDAFADAVQRARQIAAKIG | TP | 4 | Homo sapiens (Human) | |
| Q96AE4 FUBP1 FUBP1_HUMAN |
31 | 41 | VNDAFKDALQRARQIAAKIG | TP | 3 | Homo sapiens (Human) |
Please cite:
ELM-the Eukaryotic Linear Motif resource-2024 update.
(PMID:37962385)
ELM data can be downloaded & distributed for non-commercial use according to the ELM Software License Agreement
ELM data can be downloaded & distributed for non-commercial use according to the ELM Software License Agreement