| Accession: | |
|---|---|
| Functional site class: | LYPxL ESCRT motif |
| Functional site description: | The LYPxL motif binds to the V-domain of eukaryotic Alix protein. Alix is associated with the ESCRT system, which is involved in endosomal sorting of membrane proteins. Many functional instances of the motif are known from retroviruses that use the host ESCRT system to bud from the cellular plasma membrane. In viruses, the LYPxL motif comprises a viral late assembly domain (L-domain). L-domains, located in viral Gag proteins, are required for the release of virions from the host cell. Mammalian host protein Syntenin has the LYPxL motif. In fungi such as yeasts, PACC and RIM101 have the motif. |
| ELMs with same func. site: | LIG_LYPXL_L_2 LIG_LYPXL_S_1 LIG_LYPXL_SIV_4 LIG_LYPXL_yS_3 |
| ELM Description: | The LYPxL motif binds to a hydrophobic groove in the central V-domain of Alix (Lee,2007). The binding is stabilized by both hydrophobic interactions as well as by a few important hydrogen bonds. The 3D structure of the long version of the HIV-1 LYPxL motif in complex with Alix has been solved using X-ray crystallography (Zhai,2008; 2R02). The two hydrophobic residues flanking the Tyr at +2 make important hydrophobic contacts with Alix: Their substitution with alanine abrogates binding. At position +1, a leucine side chain rests against a hydrophobic surface on the Alix protein formed by residues Ala505, Gln508, Val509, Asn669 and Glu672. The second position of the motif is fully conserved, tolerating solely tyrosine. It inserts into a deep hydrophobic pocket of Alix forming a hydrogen bond between its phenolic hydroxyl and a conserved aspartate (Zhai,2008). Mutational analyses have shown that substitution of tyrosine to phenylalanine impedes binding. Proline at position +3 is also highly conserved. It leans against three hydrophobic residues on the Alix surface, with the binding centered on a conserved phenylalanine in Alix (Baietti,2012). The long version of the LYPxL motif adds another hydrophobic residue to the interaction and now adopts a 2-turn alpha helical conformation in order to fit into the limited space available at the interaction site (Sharma,2010). This extends the spacer to LYPxxxL. The new hydrophobic residue is three positions downstream of the classical motif at the end of the second helical turn. Thereby the LYPxL consensus pattern is extended to LYPxxxLxxL. The affinity of LYPLTSLRSL in HIV-1 to Alix is 40 micromolar (Zhai,2008). This together with alignment information forms the basis for the derived ELM pattern for the long version of the LYPxL motif. Exclusion of Prolines, which cannot make backbone H-bonds, is specified for the second helical turn. |
| Pattern: | [ILVM]YP...[ILVM][^P][^P][LI] |
| Pattern Probability: | 0.0000030 |
| Present in taxon: | Eukaryota |
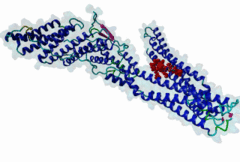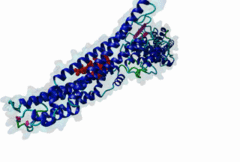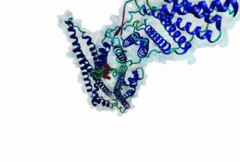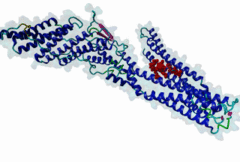
| Interaction Domain: |
ALIX_LYPXL_bnd (PF13949)
ALIX V-shaped domain binding to HIV
(Stochiometry: 1 : 1)
|
The LYPxL motif interacts with the cellular protein Alix (ALG2-interacting protein-1, or AIP1), which is associated with the Endosomal Sorting Complex Required for Transport (ESCRT). The ESCRT system is involved in the selective trafficking of membrane proteins to the lysosome by incorporating the membrane proteins into multivesicular bodies (MVBs). The final step in the biogenesis of MVBs is the ESCRT-mediated abscission of the cargo-containing vesicular membrane from the perimeter membrane (reviewed in Raiborg,2009). Some viruses have evolved strategies to hijack this process, which enables them to use ESCRT for the budding of viral particles from the host cell membrane. Short peptide sequences within viral Gag proteins (encoding the structural proteins of the virus) are required for the separation of the virus from the host cell membrane. These sequences are called late assembly (L-) domains and mediate the interaction with components of ESCRT. So far, three different linear motifs in L-domains have been extensively studied: PTAP (LIG_PTAP_UEV_1), PPxY (LIG_WW_1) and LYPxL. These L-domains can function individually as well as cooperatively, and were demonstrated to be interchangeable between different viruses (reviewed in Demirov,2004). In 1991, the importance of the PTAP residues of the caspid precursor for export of HIV-1 was reported (Gottlinger,1991). The LYPxL motif was then first characterized as a functional L-domain in EIAV by Puffer,1998. The link between the LYPxL motif and ESCRT was then established by identifying the ESCRT-associated protein Alix as a binding partner of LYPxL (Strack,2003). The recruitment of Alix is used to direct further members of ESCRT to the viral budding site, assembling the budding complex, which mediates the release of viral particles from the host cell. In this way, Alix acts as a bridging factor between ESCRT I and ESCRT III by binding both Tsg101 (ESCRT I) and CHMP4 (ESCRT III) (Pincetic,2009). LYPxL and PTAP are among the many short linear motifs that were first identified in viral proteins and only later in the regular cellular context. The importance of the LYPxL motif in viral budding varies among different viruses, depending on the presence of other L-domains. LYPxL is essential for budding if it is the only L-domain motif present, as in EIAV. In contrast, other viruses such as HIV-1 possess more complex L-domains that can include two other ESCRT related motifs PTAP and PPxY that also contribute to efficient viral budding (Bieniasz,2006). In general, the role of the LYPxL L-domain in viral budding is considered to be minor compared to PPxY and PTAP (Dilley,2010). In addition to viruses, the Apicomplexan parasite Toxoplasma gondii uses the YPxL motif during entry into the host cell: Two copies of LYPxL are present in the RON4 effector protein (Guerin,2017). The LYPxL motif binds to a hydrophobic pocket located in the central V-domain of Alix. The binding is stabilized mostly by van-der-Waals interactions as well as a few hydrogen bonds. Within the LYPxL motif, two flanking hydrophobic residues as well as the tyrosine are considered to be crucial for the binding (Zhai,2008). The number of random residues tolerated at the fourth position is restricted to either one or three amino acids. PacC, a transcription factor in Aspergillus nidulans, undergoes a two-step proteolytic activation as a response to alkaline pH. The Alix homologue PalA is required to direct a signalling protease to the cleavage sites of PacC. PacC contains two LYPxL motifs, one at each cleavage site to recruit PalA (Vincent,2003). The presence of LYPxL motifs in vertebrate cellular protein Syntenin allows its interaction with Alix, together with Syndecan, their complexes might ‘resemble’ viral budding and pertain to the formation of Intra-Luminal Vesicles (ILVs) and exosomes (Baietti,2012). Until more cellular LYPxL motifs are identified, it will be difficult to fully assess the role of the motif in vesicular trafficking of the cell. Two variant motifs - short (LIG_LYPXL_S_1) and long (LIG_LYPXL_L_2) have been mainly identified in various viruses. Two new variant motifs short yeast (LIG_LYPXL_yS_3) and long SIV (LIG_LYPXL_SIV_4) based on new cellular and viral evidence provide a broader and flexible range of sequences for identifying additional V-Domain binders. All four variants are given a separate regular expression in ELM. |
(click table headers for sorting; Notes column: =Number of Switches, =Number of Interactions)
| Acc., Gene-, Name | Start | End | Subsequence | Logic | #Ev. | Organism | Notes |
|---|---|---|---|---|---|---|---|
| P04591 gag GAG_HV1H2 |
483 | 492 | PIDKELYPLTSLRSLFGNDP | TP | 4 | HIV-1 M:B_HXB2R | |
| P35962 gag GAG_HV1Y2 |
483 | 492 | PIDKELYPLASLRSLFGSDP | TP | 9 | Human immunodeficiency virus type 1 (isolate YU2) | |
| P03347 gag GAG_HV1B1 |
495 | 504 | PIDKELYPLTSLRSLFGNDP | TP | 7 | Human immunodeficiency virus type 1 BH10 | |
| P17282 gag GAG_SIVCZ |
491 | 500 | EGESSLYPPTSLKSLFGSDP | TP | 2 | Simian immunodeficiency virus |
Please cite:
ELM-the Eukaryotic Linear Motif resource-2024 update.
(PMID:37962385)
ELM data can be downloaded & distributed for non-commercial use according to the ELM Software License Agreement
ELM data can be downloaded & distributed for non-commercial use according to the ELM Software License Agreement