| Accession: | |
|---|---|
| Functional site class: | Pex14 ligand motif |
| Functional site description: | Several linear motifs bind to a hydrophobic groove on Pex14, a key protein in peroxisomal import. Wxxx[FY] and Fxxx[FW] motifs are present in peroxisomal import receptors Pex5 (P50542) and Pex19 (P40855), respectively. The LVXEF[LM] motif is only present in the Pex5 receptor. These three motifs bind to the same hydrophobic binding site in Pex14 (O75381) which is the minimal translocon that is essential for TRG_PTS1 cargo translocation into peroxisomal matrix. The N-terminal domain of Pex14 interacts with Wxxx[FY] and LVXEF[LM] motifs in Pex5 (Fungi: LIG_Pex14_4) to target PTS1-containing peroxisomal matrix enzymes entry into the peroxisomal matrix followed by interaction between cargo-free Pex5 with SH3 domain of Pex13 (Q92968) via Wxxx[FY] motif for recycling of Pex5 into the cytosol. Pex19 contains an FxxxF motif that mediates Pex19-Pex14 interactions. Pex19 is considered to be the cytosolic import receptor for peroxisomal membrane proteins that contain an mPTS motif. |
| ELMs with same tags: |
|
| ELMs with same func. site: | LIG_Pex14_1 LIG_Pex14_2 LIG_Pex14_3 LIG_Pex14_4 |
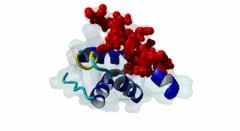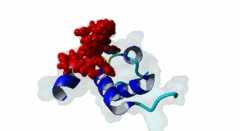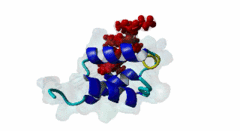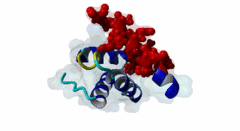
| ELM Description: | The Wxxx[FY] motif is present in the N-terminal half of soluble Pex5 protein across species including human, protozoa, yeast, arabidopsis and watermelon. Ninety percent of PEX5 proteins are present in the cytosol and ten percent in the lumen of peroxisome (Wimmer,1999). These motifs bind to Pex13 and Pex14 proteins located at the outer surface of peroxisomal and glycosomal membranes, interacting with either a SH3 domain (SM00326) of Pex13 or a conserved region within the N-terminus of Pex14 (PF04695). The first position after the tryptophan of the Wxxx[FY] motif can be any amino acid while the second and third positions have preference for hydrophilic amino acids. The third position is usually occupied by ASP, GLU or GLN (Schliebs,1999). Removal of either tryptophan at position 1 or phenylalanine/tyrosine at position 5 will result in loss of motif function. Mutations of residues at position 3 or 4 did not abolish interaction (Saidowsky,2001). Based on secondary structure predictions (Schliebs,1999) and crystal structure data (2W84, Neufeld,2009), this motif is part of an amphipathic alpha-helix. Upon binding to Pex14, Pex5 adopts an amphipathic α-helical conformation with a negatively charged surface in Pex5 binding to a positively charged Pex14 surface. It was suggested that charge complimentarity is one of the determinants of Pex5-Pex14 interaction. The helices α1 and α2 of Pex14, to which Pex5 binds, are antiparallel whereas the α3 helix is diagonal across α1 and α2. A major determinant of affinity is the requirement for the segment that contains the Wxxx[FY] motif to being able to form an α-helix, while a negatively charged amino acid at the ninth position from tryptophan reduces binding affinity (Neufeld,2009). |
| Pattern: | W...[FY] |
| Pattern Probability: | 0.0002226 |
| Present in taxon: | Eukaryota |
| Interaction Domains: |
|
Peroxisomes are single-membrane spherical subcellular organelles found in eukaryotes. Peroxisomes belong to the microbody family of organelles along with glyoxysomes found in plants and glycosomes found in trypanosomes. The peroxisomal matrix contains enzymes involved in hydrogen peroxide metabolism, alpha and beta oxidation of long chain fatty acids, branched chain fatty acids, D-amino acids, and polyamines. Peroxisomes also synthesise cholesterol, bile acids and ether lipids (plasmalogens) in mammals (Lanyon-Hogg,2010, Ma,2011). The import of peroxisomal matrix enzyme proteins (termed PTS1 cargo) into the peroxisome involves recognition of the PTS1 cargo by a Pex5 receptor in the cytosol, docking of PTS1-Pex5 complex at peroxisomal membrane, and translocation of the PTS1 cargo across the peroxisomal membrane into the matrix. This is followed by Pex5 receptor recycling back into the cytosol for another round of PTS1 cargo import. Unfolded, folded, oligomeric and cofactor-bound proteins are imported into peroxisome (Pires,2003). Most peroxisomal matrix proteins carry either PTS1 (TRG_PTS1) or PTS2 (TRG_PTS2) signals at the C-terminus or N-terminus of proteins, respectively. Pex5 cytosolic receptor recognises PTS1 signal and Pex7 cytosolic receptor recognises PTS2 signal. The majority of peroxisomal matrix proteins carry the PTS1 sequence. PTS1 signals are similar to the canonical terminal sequence SKL$. Variant examples of the PTS1 signal are PRM$ in multifunctional beta-oxidation protein MFP II from cucumber; SRM$ and ARM$ in isocitrate lyase protein in oilseed rape, tomato, cottonseed; SKL$ and SRL$ in malate synthase protein in pumpkin, cottonseed, and castor bean; ARF$ in H. polymorpha alcohol oxidase (Wimmer,1999). PTS1-carrying cargo are recognised by the cytosolic receptor Pex5. Pex5 is a two domain protein, composed of a highly conserved C-terminal half consisting of 6-7 tetratricopeptide repeats (TPR) (PF00515 and 13414), and a poorly conserved and natively disordered N-terminal half (PF04695) including multiple Wxxx[FY] motifs and sequences that are required for Pex5 recycling. These TPR repeats are essential for the binding of Pex5 to PTS1 cargo but there are other contacts between the cargo and Pex5 beside TPR repeats. Cytosolic cargo-free Pex5 was reported to be a monomer (Costa-Rodrigues,2005) whereas PTS1-bound Pex5 is dimeric protein (Madrid,2004). In the unbound state, N- and C-terminal regions of Pex5 interact with each other, rendering TPR repeats inaccessible to PTS1 cargo. There is opposing evidence of whether heat-shock protein 70 (Hsp70) participates in cargo binding by producing a conformational change in Pex5 to open up the receptor (evidence for Hsp70 involvement, Harano,2001; evidence against Hsp70 involvement, Harper,2003). Pex5 TPR domain undergoes conformational change from open, snail-like conformation into a closed, ring-like conformation when it binds to PTS1 cargo (Stanley,2006). Pex5-PTS1 heterodimeric complex interacts with the docking complex at the peroxisomal membrane. The docking complex is comprised of Pex13, Pex14, and Pex17 which is not conserved in all organisms. Pex8 is part of the docking complex in yeasts. The release of the PTS1 cargo from the Pex5 receptor and translocation of PTS1 cargo into peroxisomal matrix is an incompletely understood process. Evidence suggests that cargo-bound Pex5 interacts with the N-terminal region of Pex14 via Wxxx[FY] motifs as a targeting step to peroxisomal membrane. FxxxW motif in S. cerevisiae Pex5 was shown to bind to Wxxx[FY]-binding site in Pex14. FxxxW motif however bound in an inverted conformation. A similar motif FxxxF was found in human Pex19 protein and is capable of interacting with Wxxx[FY]-binding site in Pex14. It also binds in an inverted conformation to Wxxx[FY] motif. Pex19 interaction with conserved N-terminal region of Pex14 via FxxxF motif is 130-fold weaker than Pex5-Pex14 interaction (Neufeld,2009). The function of Pex19-Pex14 interaction is presently unknown. The affinity of Pex5 for PTS1 cargo is decreased during Pex5 interaction with Pex14. Affinity studies demonstrated that cargo-loaded Pex5 favours interaction with Pex14 whereas cargo-free Pex5 has higher affinity for Pex13 (Urquhart,2000). Cargo-free Pex5 Wxxx[FY] motif interacts with SH3 domain (SM00326) of Pex13 after the release of PTS1 cargo into peroxisomal matrix. Wxxx[FY] motif in Pex5 represents a novel binding site for SH3 domain of pex13. Avidity binding between Pex5 and Pex14 takes place where multiple Pex14 proteins bind to a single Pex5 receptor due to presence of multiple Wxxx[FY] motifs in Pex5. The affinity of Pex5 for PTS1 cargo is decreased during Pex5 interaction with Pex14 and the newly characterized LVAEF-binding motif found in the N-terminus of human Pex5 directs the binding of Pex14-NTD to Pex5 with a faster binding kinetics than the binding via Wxxx[FY] motifs. The LVAEF motif represents a docking site for cargo loaded receptor and it assist in establishing the first contact of Pex14 with PTS1 receptor. Mutating motif to alanines affects the import of proteins into peroxisomes. Evolutionary conserved consensus sequence of the motif is LVXEF[LM] while only in fungi it is MM[NDE][EDG]F[LM] (Neuhaus,2014). Since concrete evidence is lacking for PTS1 cargo translocation and release, three models explaining translocation and release of PTS1 cargo into peroxisomal matrix have been proposed. The extended shuttle model proposes that receptor-cargo complex completely enters the peroxisomal lumen for cargo unloading. Simple shuttle model states that the receptor is partially exposed to the peroxisomal lumen before unloading the cargo. Transient pore model proposes that a population of Pex5 inserts into the membrane forming a pore through which the receptor-cargo complex can pass (Lanyon-Hogg,2010). Evidence for the dynamic pore comes from two studies. One study utilised gold particles (Walton,1995) whereas another study used Fox1 cargo (Meinecke,2010). Both studies showed that the size of the pore can increase up to 9 nm, large enough to facilitate oligomerized cargo entry into peroxisomal matrix. Absence of Pex5 resulted in no pore-forming activity indicating that Pex5 is a requirement for transient pore formation. Pex5 was shown to be a constituent of the pore during PTS1 cargo entry (Meinecke,2010). Recycling of Pex5 back into the cytosol requires monoubiquitination that occurs on a conserved cysteine residue near N-terminus of Pex5 with the formation of thioester bond between the conserved cysteine of Pex5 and ubiquitin (Grou,2008, Williams,2007). The Wxxx[FY] motif is also required for PTS2 cargo import into peroxisomal matrix. In the cytosol, Pex7 receptor interacts with PTS2 sequence located in N-terminus of the cargo. The PTS2-Pex7 complex alone is insufficient to bind to the docking complex at peroxisomal membrane. Pex7 co-receptors, Pex18, Pex20, or Pex21 present in yeast are required. In mammals and plants, homologues of these proteins are not found. Instead long isoform of Pex5 (Pex5L) serves as co-receptor for Pex7 (Schliebs,2006). Pex5L differs from short isoform of Pex5 (Pex5S) by insertion of 37 amino acids in Pex5L. The common feature of mammalian Pex5L and yeast Pex18, Pex20, and Pex21 is the presence of Wxxx[FY] motif. Wxxx[FY] motif in P. pastoris Pex20 was shown to bind to N-terminal region of Pex14 (Leon,2006). Pex7 co-receptors bind to Pex7 and direct tertiary complex to Pex14 docking factor at peroxisomal membrane. Hence, the Wxxx[FY] motif participates in docking, translocation, and release of PTS1 and PTS2 peroxisomal matrix enzymes. |
(click table headers for sorting; Notes column: =Number of Switches, =Number of Interactions)
Please cite:
ELM-the Eukaryotic Linear Motif resource-2024 update.
(PMID:37962385)
ELM data can be downloaded & distributed for non-commercial use according to the ELM Software License Agreement
ELM data can be downloaded & distributed for non-commercial use according to the ELM Software License Agreement