LIG_TRAF3_MATH_PxP_3
| Accession: | |
|---|---|
| Functional site class: | TRAF2 group (TRAF1,2,3,5) MATH domain binding motifs |
| Functional site description: | Ring finger E3 ligase TRAF2 specifically interacts with TNF receptor superfamily members and connects the receptors to downstream signalling proteins. The receptor binding groove of the TRAF2 MATH domain is similar to that of TRAF1, 3 and 5, but dissimilar to those of TRAF4 and 6. TRAF2 forms complexes with other RING E3 ligases cIAP1/2 for Lys63-linked polyubiquitination and NF-κB activation. The Lys63-Ub chains are not signals for destruction so this motif is not a degron. This process is central to the NF-κB gene activation pathways based on surface receptor signalling. Bound TNFRs, including CD30, CD40, CD27 and Ox40, elicit cellular processes involved in developmental, immunological and inflammatory signalling. There are at least two types of PxQ-based TRAF2-like-binding motifs (short or “major” and long or “minor”). TRAF3 has a specific PxP-based variant in addition to binding the shared motifs. The TRAF2-binding motif is mimicked in the proteins of some pathogens, such as Epstein-Barr virus. |
| ELMs with same func. site: | LIG_TRAF2like_MATH_loPxQ_2 LIG_TRAF2like_MATH_shPxQ_1 LIG_TRAF3_MATH_PxP_3 |
| ELM Description: | As with other TRAF2-group members, TRAF3 can recognise PxQ-type motifs. Additionally, TRAF3 can recognise motifs with PxP at +1 and +3. The PxP variant motif that specifically binds TRAF3 was identified in the B-cell-activating factor receptor (BAFF-R, also named TNFRSF13C), a member of the TNFR superfamily (Ni,2004). Structural studies of the BAFF-R:TRAF3 complex (2GKW) shows that the BAFF-R PVPATE sequence binds on the same crevice that other TRAF motifs bind, stacking on F448. Pro at +1 binds at the same surface as the PxQ TBM motifs. The +2 position makes backbone β-augmentation to the MATH β-strand 7, and its sidechain is always bulky, probably shielding the backbone H-bonding. Pro is not allowed at this position. Pro at +3 packs onto S468 (but does not interact with the other two key Ser positions as does Gln at +3). Ala at +4 packs on F410. Thr at +5 H-bonds to D399. Glu at +6 makes a charged interaction with R393. A second PxP TBM structure (1RF3) has been reported for the lymphotoxin β receptor (TNFR3). In the BAFF-R structure, there is extended binding after the core motif by several residues (2GKW) as is seen in some other TBM structures but here the sequence seems weakly conserved and has not been included in the motif pattern. |
| Pattern: | P[ILVT]P(([ED][^P].)|(.[^P][ED])) |
| Pattern Probability: | 0.0004595 |
| Present in taxon: | Vertebrata |
| Interaction Domain: |
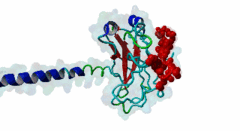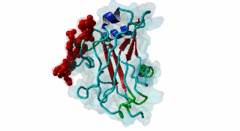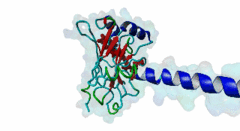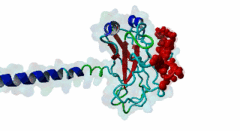
TRAF3, MATH domain (IPR037304)
Tumor necrosis factor (TNF) receptor associated factor 3 (TRAF3) is a highly versatile regulator that positively controls type I interferon production, but negatively regulates mitogen-activated protein (MAP) kinase activation and non-canonical nuclear factor-kB signalling
(Stochiometry: 1 : 1)
|
The Tumour necrosis factor Receptor-Associated Factors (TRAFs) are a family of seven Ring-type E3 ligases, most of which have a C-terminal MATH domain that interacts with substrates containing the appropriate SLiM (Yamamoto,2021), which has been termed TBM (TRAF-Binding Motif). The TRAF family is named for its association with members of the TNFR membrane receptor superfamily (Rothe,1994), but TRAFs also interact with numerous cytosolic proteins such as the TANK adaptor protein (Q92844). TRAF proteins play critical roles in the transmission of signals from the surface receptors leading to the activation of NF-κB gene expression. TRAF6, along with 2 and 5, acts in the canonical activation pathway, while TRAF2 and 3 act in the non-canonical pathway (Yamamoto,2021). A striking feature of TRAF6 cell biology is that the sets of interacting proteins may be completely different in the various signalling systems in which it participates (Yamamoto,2021; Chathuranga,2021; Dainichi,2019). TRAF1,2,3 and 5 MATH domains share the highest sequence identity to one another and may have evolved through gene duplication subsequent to earlier divergence from the TRAF4 and 6 homologues (Foight,2016). The sequence identity trend holds similarly among TRAF1,2,3 and 5 when the core peptide binding site is considered (Foight,2016). Different binding motifs have been defined for TRAFs1,2,3 and 5 versus TRAF4 and 6. The residues in the three binding hotspots of TRAF1,2,3 and 5 are highly conserved, indicating they share many common substrates with a similar mode of interaction (Park,2018). There are two variant motifs for TRAF2 binding, mainly based on the length. The short motif with consensus PxQE has been termed the major motif, and the longer motif with consensus PxQxxD as the minor motif. Many of the TNF-R family members like CD30, CD40, OX40, CD27 and 4-1BB (CD137) contain the shorter motif. DYRK1A is a newly identified binding partner of TRAF2 that contains the short motif (Zhang,2021). TANK (also known as I-TRAF) possesses a C-terminally extended motif (1L0A; 1KZZ). It acts as an inhibitor of TRAF function by competitively binding versus the TRAF2 binding motifs in CD40, TNFR2 and EBV LMP1. The LMP1 protein of Epstein-Barr virus also contains a longer motif that is involved in NF-kB activation (Kaye,1996). Various studies have shown that many of the TRAF2 binding sites are also recognised by other TRAFs like TRAF1,3 and 5 with different affinity and that is important for their different functions in TRAF-mediated signal transduction (Foight,2016). SPOT arrays confirm the overall similarity of the short motif preferences for this group (Pullen,1999). In addition to recognising PxQ motifs, unlike TRAFs1, 2 and 5, TRAF3 can also uniquely recognise a PxP variant motif found in BAFF-R and TNFR3 (Ni,2004; Li,2003). |
-
Structurally distinct recognition motifs in lymphotoxin-beta receptor and CD40 for tumor necrosis factor receptor-associated factor (TRAF)-mediated signaling.
Li C, Norris PS, Ni CZ, Havert ML, Chiong EM, Tran BR, Cabezas E, Reed JC, Satterthwait AC, Ware CF, Ely KR
J Biol Chem 2003 Dec 08; 278 (50), 50523-9
PMID: 14517219
-
Key molecular contacts promote recognition of the BAFF receptor by TNF receptor-associated factor 3: implications for intracellular signaling regulation.
Ni CZ, Oganesyan G, Welsh K, Zhu X, Reed JC, Satterthwait AC, Cheng G, Ely KR
J Immunol 2004 Dec 15; 173 (12), 7394-400
PMID: 15585864
(click table headers for sorting; Notes column: =Number of Switches, =Number of Interactions)
| Acc., Gene-, Name | Start | End | Subsequence | Logic | #Ev. | Organism | Notes |
|---|---|---|---|---|---|---|---|
| P36941 LTBR TNR3_HUMAN |
387 | 392 | ATPEPPYPIPEEGDPGPPGL | TP | 2 | Homo sapiens (Human) | |
| Q96RJ3 TNFRSF13C TR13C_HUMAN |
162 | 167 | TPPGHSVPVPATELGSTELV | TP | 2 | Homo sapiens (Human) |
Please cite:
ELM-the Eukaryotic Linear Motif resource-2024 update.
(PMID:37962385)
ELM data can be downloaded & distributed for non-commercial use according to the ELM Software License Agreement
ELM data can be downloaded & distributed for non-commercial use according to the ELM Software License Agreement