LIG_EVH1_2
| Accession: | |
|---|---|
| Functional site class: | EVH1 ligands |
| Functional site description: | Proline-rich sequences that bind to the signal transduction modules EVH1. |
| ELMs with same func. site: | LIG_EVH1_1 LIG_EVH1_2 LIG_EVH1_3 |
| ELM Description: | Proline-rich motif binding to signal transduction class II EVH1 domains. |
| Pattern: | PP..F |
| Pattern Probability: | 0.0001632 |
| Present in taxons: | Bos taurus Caenorhabditis elegans Drosophila melanogaster Eumetazoa Homo sapiens Mus musculus Oryctolagus cuniculus Rattus norvegicus Sus scrofa Xenopus laevis |
| Interaction Domain: |
WH1 (PF00568)
WH1 domain
(Stochiometry: 1 : 1)
|
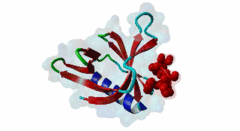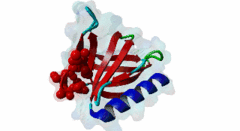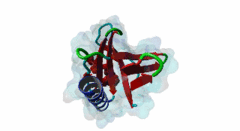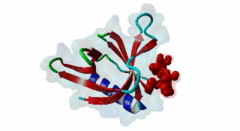
Enabled/vasodilator-stimulated phosphoprotein homology 1 (EVH1) domains are 115 residue protein-protein interaction modules which provide essential links for their host proteins to various signal transduction pathways. Many EVH1-containing proteins are associated closely with actin-based structures and are involved in re-organization of the actin cytoskeleton. EVH1 domains are also present in proteins enriched in neuronal tissue, thus implicating them as potential mediators of synaptic plasticity, linking them to memory formation and learning. EVH1 domains recognize and bind specific proline-rich sequences (PRSs). In general, a small (3-6 residue) core PRS in the target protein binds a 'recognition pocket' on the domain surface. Further affinity- and specificity-increasing interactions are then formed between additional domain epitopes and peptide 'core-flanking' residues. The protein-protein interactions mediated by EVH1 domains are clearly highly important for the regulation of signal transduction events, re-organization of the actin cytoskeleton, and modulation of actin dynamics and actin-based motility. EVH1 domains can be divided into three classes based on the consensus sequences of their target PRS ligands. Single residue peptide substitution experiments have shown the consensus binding motif for class I EVH1 domains to be F-P-X-(hydrophobic)-P. Such sequences are found in a number of mammalian proteins, including the focal adhesion proteins zyxin and vinculin, as well as in the ActA protein of the intracellular pathogen Listeria monocytogenes. Class II EVH1 domains are found in the Homer-Vesl protein family of postsynaptic receptor associated proteins. These recognize a consensus PRS, with a core motif comprising PPxxF, found in the group I mGluRs, IP3Rs, RyRs and the Shank family proteins. The EVH1 domains exclusive for WASP and N-WASP proteins are also called WH1/ WASP-homology 1 domains. The EVH1 class III motifs are found in the verprolin family of proteins. They are known to play a role in WASP/N-WASP- mediated processes (e.g. regulation actin filament dynamics by WIP and WIRE), yet their exact functions remain to be elucidated. The recognition motif of EVH1 class III includes a polyproline region, which is a common feature of all EVH1 motifs, yet in class III it comprises an additional region which contains two highly conserved phenylalanines. |
-
Homer binds a novel proline-rich motif and links group 1 metabotropic
glutamate receptors with IP3 receptors.
Tu JC, Xiao B, Yuan JP, Lanahan AA, Leoffert K, Li M, Linden DJ, Worley PF
Neuron 1998 Oct; 21 (4), 717-26
PMID: 9808459
-
Structure of the enabled/VASP homology 1 domain-peptide complex: a key
component in the spatial control of actin assembly.
Prehoda KE, Lee DJ, Lim WA
Cell 1999 May 14; 97 (4), 471-80
PMID: 10338211
-
Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of
postsynaptic density proteins.
Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, Brakeman P, Doan A, Aakalu VK, Lanahan AA, Sheng M, Worley PF
Neuron 1999 Jul; 23 (3), 583-92
PMID: 10433269
-
Structure of the Homer EVH1 domain-peptide complex reveals a new twist in
polyproline recognition.
Beneken J, Tu JC, Xiao B, Nuriya M, Yuan JP, Worley PF, Leahy DJ
Neuron 2000 Apr; 26 (1), 143-54
PMID: 10798399
-
An EVH1/WH1 domain as a key actor in TGFbeta signalling.
Callebaut I
FEBS Lett 2002 May 22; 519 (1), 178-80
PMID: 12023040
-
Crystal structure of the Homer 1 family conserved region reveals the
interaction between the EVH1 domain and own proline-rich motif.
Irie K, Nakatsu T, Mitsuoka K, Miyazawa A, Sobue K, Hiroaki Y, Doi T, Fujiyoshi Y, Kato H
J Mol Biol 2002 May 10; 318 (4), 1117-26
PMID: 12054806
(click table headers for sorting; Notes column: =Number of Switches, =Number of Interactions)
| Acc., Gene-, Name | Start | End | Subsequence | Logic | #Ev. | Organism | Notes |
|---|---|---|---|---|---|---|---|
| Q9JLU4 Shank3 SHAN3_RAT |
908 | 912 | EVGDVPRPAPAATPPERPKR | TP | 2 | Rattus norvegicus (Norway rat) | |
| Q9JLU4 Shank3 SHAN3_RAT |
1385 | 1389 | STVSSMSTLSSESGELTDTH | TP | 4 | Rattus norvegicus (Norway rat) | |
| P21817 RYR1 RYR1_HUMAN |
1773 | 1777 | GVTTSLRPPHHFSPPCFVAA | TP | 4 | Homo sapiens (Human) | |
| P31424 Grm5 GRM5_RAT |
1156 | 1160 | EELVALTPPSPFRDSVDSGS | TP | 5 | Rattus norvegicus (Norway rat) | |
| Q13255 GRM1 GRM1_HUMAN |
1147 | 1151 | DDSPALTPPSPFRDSVASGS | TP | 1 | Homo sapiens (Human) | |
| P23385 Grm1 GRM1_RAT |
1152 | 1156 | EDSPALTPPSPFRDSVASGS | TP | 5 | Rattus norvegicus (Norway rat) | |
| Q14643 ITPR1 ITPR1_HUMAN |
49 | 53 | ETGDLNNPPKKFRDCLFKLC | TP | 1 | Homo sapiens (Human) | |
| Q08877 Dnm3 DYN3_RAT |
809 | 813 | PGPHSGAPPVPFRPGPLPPF | TP | 1 | Rattus norvegicus (Norway rat) |
Please cite:
ELM-the Eukaryotic Linear Motif resource-2024 update.
(PMID:37962385)
ELM data can be downloaded & distributed for non-commercial use according to the ELM Software License Agreement
ELM data can be downloaded & distributed for non-commercial use according to the ELM Software License Agreement