DOC_SPAK_OSR1_1
| Accession: | |
|---|---|
| Functional site class: | SPAK-OSR1 docking motif |
| Functional site description: | The Ste20-related proline alanine-rich kinase (SPAK) and oxidative stress response kinase (OSR1) are the only two mammalian serine-threonine kinases belonging to the germinal centre-like kinase subfamily VI. The CCT domain of SPAK/OSR1 proteins represents a novel protein fold called SPOC. The CCT domains of human SPAK-OSR1 are 79% identical at the sequence level and are also highly conserved in other metazoan organisms. The main described function of the CCT domain SPOC fold is to interact with a particular docking site called the RFxV motif in order to bind and phosphorylate the target proteins. The RFxV motif is also found in the upstream activating kinases WNK1 and WNK4 and so it is also used to dock these activating kinases to their SPAK/OSR1 substrates. |
| ELM Description: | The SPAK and OSR1 kinases specifically recognize their upstream activators and downstream substrates by interacting with their RFxV motifs. The Conserved C-Terminal (CCT) domain of SPAK and OSR1 acts as a binding domain for this interaction. The primary pocket of the CCT domain forms a web of molecular interactions with the Arg, Phe and Val residues of the RFxV-containing peptide and mutation of any of the conserved residues prevents the interaction. Ile is allowed at the V position. Proline is not allowed at the X position due to the backbone contacts it makes in the beta augmentation with the edge beta strand of the CCT. The motif has so far only been found in the Metazoa. |
| Pattern: | RF[^P][IV]. |
| Pattern Probability: | 0.0000768 |
| Present in taxon: | Metazoa |
| Interaction Domain: |
OSR1_C (PF12202)
Oxidative-stress-responsive kinase 1 C terminal
(Stochiometry: 1 : 1)
|
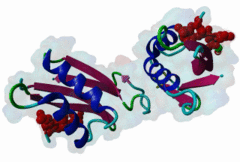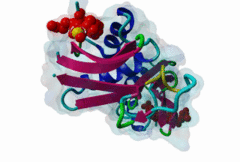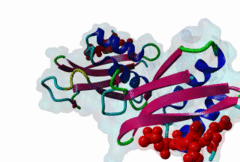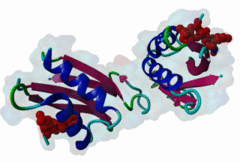
OSR1 (oxidative stress-responsive-1) and SPAK (Ste20/Sps1-related proline/alanine-rich kinase) are related kinases belonging to the GCK-VI subfamily of Ste20 group kinases (Lee,2009). They phosphorylate clusters of threonine residues in their best known substrates the Na+ Cl- cotransporters NCC and NKCC2. Both SPAK and OSR1 possess similar architectures: the kinase catalytic domain, followed by a flexible region and then a conserved C-terminal (CCT) domain (Vitari,2006). SPAK has a flexible N-terminal region with an Ala-Pro stretch thought to be a positional targeting module. The CCT domains of OSR1 and SPAK are 79% identical at their sequence level and form a unique fold called SPOC (SPak/Osr1 C-terminus) fold (Villa,2007). The CCT domain is involved in the interactions of these kinases - not just with their substrates but also their activator kinases - by recognizing the docking motif RFxV (Piechotta,2002), which these all possess. Both SPAK and OSR1 are involved in many cellular process including cell differentiation, cytoskeletal rearrangement, cell proliferation, transformation and most notably are involved in the regulation of ion homeostasis and volume control in mammalian cells. Since mutations in their WNK1 and WNK4 upstream-activating kinases can cause Gordon's syndrome, an inherited disregulation of Na-Cl co-transporters, SPAK/OSR1 are also considered to be a potential therapeutic target for hypertension. Inhibition of these enzymes reduces the activity of the substrates NCC and NKCC2 and thereby reduces renal salt re-absorption and consequent lowering of blood pressure (Richardson,2008). The structure of the CCT domain consists of four antiparallel beta-strands packed against two large and one smaller alpha helices (2V3S) (Villa,2007). The interface between alpha1 and beta2 creates an elongated, negatively charged groove called the primary pocket while a secondary hydrophobic pocket is formed by the large loop connecting alpha3 and beta4. The CCT domain interacts with its docking motif via the primary pocket with the residues involved in the interaction being identical in both SPAK and OSR1. RFxV motifs with a following Thr/Ser residue are sensitive to inhibition of binding by phosphorylation which causes a steric clash between the phosphate group and the backbone of the CCT domain. It is not yet clear if SPAK and OSR1 kinase activities can themselves autoregulate RFxV interactions. |
-
Cation chloride cotransporters interact with the stress-related kinases
Ste20-related proline-alanine-rich kinase (SPAK) and oxidative stress
response 1 (OSR1).
Piechotta K, Lu J, Delpire E
J Biol Chem 2002 Dec 27; 277 (52), 50812-9
PMID: 12386165
-
Characterization of the interaction of the stress kinase SPAK with the
Na+-K+-2Cl- cotransporter in the nervous system: evidence for a
scaffolding role of the kinase.
Piechotta K, Garbarini N, England R, Delpire E
J Biol Chem 2003 Dec 26; 278 (52), 52848-56
PMID: 14563843
-
Functional interactions of the SPAK/OSR1 kinases with their upstream
activator WNK1 and downstream substrate NKCC1.
Vitari AC, Thastrup J, Rafiqi FH, Deak M, Morrice NA, Karlsson HK, Alessi DR
Biochem J 2006 Jul 1; 397 (1), 223-31
PMID: 16669787
-
SPAK and OSR1, key kinases involved in the regulation of chloride
transport.
Delpire E, Gagnon KB
Acta Physiol (Oxf) 2006 May-Jun; 187 (1), 103-13
PMID: 16734747
-
WNK1 and OSR1 regulate the Na+, K+, 2Cl- cotransporter in HeLa cells.
Anselmo AN, Earnest S, Chen W, Juang YC, Kim SC, Zhao Y, Cobb MH
Proc Natl Acad Sci U S A 2006 Jul 18; 103 (29), 10883-8
PMID: 16832045
-
Genome-wide analysis of SPAK/OSR1 binding motifs.
Delpire E, Gagnon KB
Physiol Genomics 2007 Jan 17; 28 (2), 223-31
PMID: 17032814
-
Structural insights into the recognition of substrates and activators by
the OSR1 kinase.
Villa F, Goebel J, Rafiqi FH, Deak M, Thastrup J, Alessi DR, van Aalten DM
EMBO Rep 2007 Sep; 8 (9), 839-45
PMID: 17721439
-
Activation of the thiazide-sensitive Na+-Cl- cotransporter by the
WNK-regulated kinases SPAK and OSR1.
Richardson C, Rafiqi FH, Karlsson HK, Moleleki N, Vandewalle A, Campbell DG, Morrice NA, Alessi DR
J Cell Sci 2008 Mar 1; 121, 675-84
PMID: 18270262
-
The regulation of salt transport and blood pressure by the WNK-SPAK/OSR1
signalling pathway.
Richardson C, Alessi DR
J Cell Sci 2008 Oct 15; 121, 3293-304
PMID: 18843116
-
Crystal structure of domain-swapped STE20 OSR1 kinase domain.
Lee SJ, Cobb MH, Goldsmith EJ
Protein Sci 2009 Feb; 18 (2), 304-13
PMID: 19177573
(click table headers for sorting; Notes column: =Number of Switches, =Number of Interactions)
Please cite:
ELM-the Eukaryotic Linear Motif resource-2024 update.
(PMID:37962385)
ELM data can be downloaded & distributed for non-commercial use according to the ELM Software License Agreement
ELM data can be downloaded & distributed for non-commercial use according to the ELM Software License Agreement